History of Lyophilization
 Lyophilization, commonly referred to as freeze-drying, has existed in its simplest form since as early as the 13th century. The Peruvian Incas developed the technique, originally used as a method of food preservation. Potatoes and other crops were preserved by placing them on the high mountains of the Andes, like those around Machu Picchu. This environment, with its low pressure and temperature, could be considered the first ever “freeze-dryer.”
Lyophilization, commonly referred to as freeze-drying, has existed in its simplest form since as early as the 13th century. The Peruvian Incas developed the technique, originally used as a method of food preservation. Potatoes and other crops were preserved by placing them on the high mountains of the Andes, like those around Machu Picchu. This environment, with its low pressure and temperature, could be considered the first ever “freeze-dryer.”
The first major scientific development occurred in the 1880s when Richard Altmann developed the methodological process of lyophilizing tissue for histological sections. However, Altmann’s technique went virtually unnoticed for over 40 years. In 1906, Jacques-Arsène d’Arsonval and Frédéric Bordas worked together to develop the first modern lyophilizer. Leon Shackell improved on d’Arsonval and Bordas’ lyophilizer in 1909, in which Shackell designed an electrically driven vacuum pump instead of the original lyophilizer which utilized the displacement of air with ethyl ether to produce the necessary vacuum lyophilization process.
The method of lyophilization, as applicable today, began most notably in 1933 by Earl W. Flosdorf and Stuart Mudd. Flosdorf and Mudd successfully utilized lyophilization to preserve human serum. In 1935, Flosdorf and Ronald I. N. Greaves created the first commercial lyophilizer. During World War II, Howard Walter Florey and Ernst Boris Chain utilized this technology to preserve penicillin and blood plasma, allowing invaluable medical supplies to reach remote areas. During the smallpox epidemic of the 1940s, a lyophilized vaccine under the commercial name “Dry Vax” was introduced in several countries for mass immunization. In 1960, a lyophilized rabies vaccine was successfully deployed, advancing future medical treatment.
 In the following years, due to the industrial adoption of tray-type lyophilizers which continued to improve the shelf life of products, lyophilization became more widely adopted. The term lyophilization is generally attributed to L.R. Rey. In 1960, Rey coined the term by illustrating the permeable quality of the dehydrated product and its "lyophil" attributes, as products subjected to lyophilization can quickly reabsorb a solvent, returning the substance to its initial liquid state. In the same decade, the lyophilizer became automated which allowed for more precise control over the process. Later in 1966, both NASA and Calbiochem (now EMD Chemicals) filed notable patents for the use of lyophilization for enzymes, reagents, and methods for use in testing biological samples. Several more patents were granted throughout the 1990s to early 2000s. Inventor Paul Hemmes as well as Abaxis received patents for the technical application of lyophilized bead formation with a cryogenic apparatus, which still heavily influences the lyophilization industry today. Considering additional advancements in the technology over the last 50 years, such innovations have given the foundation of EVIKTM Know-How establishing Evik Diagnostics as the lyophilization experts in the laboratory reagent industry.
In the following years, due to the industrial adoption of tray-type lyophilizers which continued to improve the shelf life of products, lyophilization became more widely adopted. The term lyophilization is generally attributed to L.R. Rey. In 1960, Rey coined the term by illustrating the permeable quality of the dehydrated product and its "lyophil" attributes, as products subjected to lyophilization can quickly reabsorb a solvent, returning the substance to its initial liquid state. In the same decade, the lyophilizer became automated which allowed for more precise control over the process. Later in 1966, both NASA and Calbiochem (now EMD Chemicals) filed notable patents for the use of lyophilization for enzymes, reagents, and methods for use in testing biological samples. Several more patents were granted throughout the 1990s to early 2000s. Inventor Paul Hemmes as well as Abaxis received patents for the technical application of lyophilized bead formation with a cryogenic apparatus, which still heavily influences the lyophilization industry today. Considering additional advancements in the technology over the last 50 years, such innovations have given the foundation of EVIKTM Know-How establishing Evik Diagnostics as the lyophilization experts in the laboratory reagent industry.
Although the general process of lyophilization remains much the same as it did atop Machu Picchu, the scientific and engineering breakthroughs mentioned above have allowed for the continued advancement in preservation technology that we at Evik Diagnostics use today. Most current production operations use advanced lyophilization equipment with extensive data acquisition and control systems to achieve consistent processes and large production throughput. That said, Machu Picchu still has us beat in the view department.
Science of Lyophilization
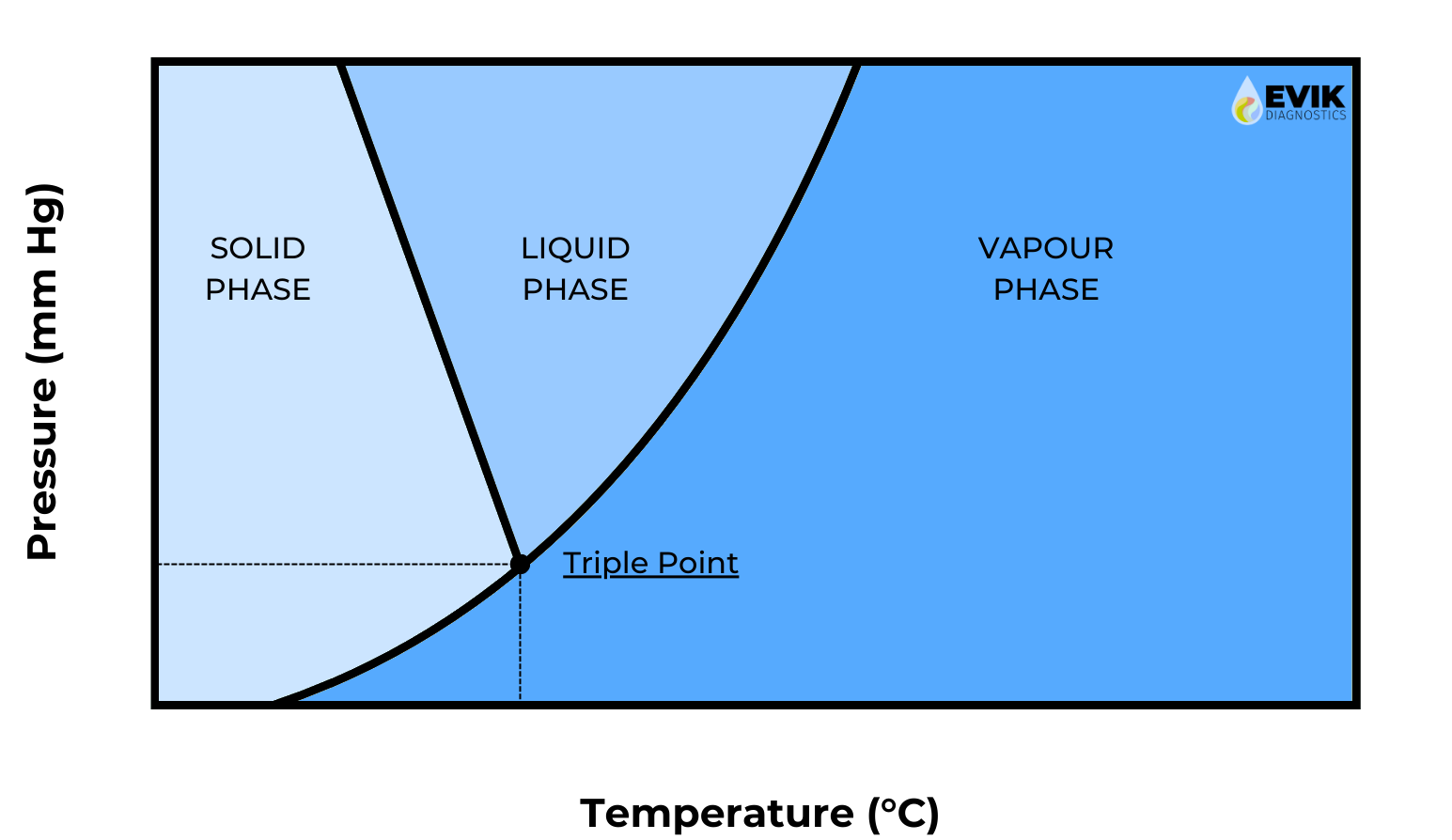 Lyophilization (LYO) is the removal of water and other solvents from liquid formulations, by freezing, then sublimation. It is an important manufacturing process in the biotechnology, diagnostics, pharmaceutical, and food and beverage industries, as the removal of water can stabilize the components allowing for long-term ambient temperature storage and increase shelf life. This reduces the logistical complexity and associated costs with cold chain shipping and storage. By first freezing, then dehydrating via the sublimation of water, lyophilization maintains the shape and structure the material had in its frozen state. At Evik Diagnostics, our expertise is harnessing these qualities to make stable and robust lyobeads for diagnostic assays.
Lyophilization (LYO) is the removal of water and other solvents from liquid formulations, by freezing, then sublimation. It is an important manufacturing process in the biotechnology, diagnostics, pharmaceutical, and food and beverage industries, as the removal of water can stabilize the components allowing for long-term ambient temperature storage and increase shelf life. This reduces the logistical complexity and associated costs with cold chain shipping and storage. By first freezing, then dehydrating via the sublimation of water, lyophilization maintains the shape and structure the material had in its frozen state. At Evik Diagnostics, our expertise is harnessing these qualities to make stable and robust lyobeads for diagnostic assays.
The basic principle of lyophilization is to freeze a solution followed by reducing the pressure below the triple-point of water. Thus, water can transition directly from the solid phase to the vapor phase, such that the structural integrity of the frozen material is maintained throughout the process. Lyophilization consists of three major steps: freezing, primary drying, and secondary drying. After the product is frozen, the chamber pressure of the freeze-dryer is reduced so sublimation begins. Primary drying is the most time-consuming part of the LYO process. Primary drying is complete when all ice has sublimated. At this point, there may still be a large fraction of water present in the product. However, rather than existing in a solid phase, this water is adsorbed to other molecules in the product. This adsorbed water may have a harmful effect on the stability of the active ingredient, so it is often important to remove as much as possible. This is achieved through secondary drying. With the ice removed from the product, it is now safe to increase the product temperature. Increasing the product temperature (~ 30 °C) speeds up the desorption rate significantly. This takes a few hours, and once completed, the moisture content of the product is around 0-3 %.
 Developing a formulation for lyophilization must account for several important factors. First, stability of the active ingredients must be considered, especially when it comes to biomolecules. For example, it may be important to work within specific pH ranges to maintain functionality for certain components. Besides functional considerations, critical final product quality attributes such as appearance, color, robustness, solubility, and residual moisture must also be considered. Finally, scientists must consider the thermal properties of a formulation ensuring its glass transition temperature or eutectic temperature is in a range suitable for LYO. To meet these targets, excipients are carefully selected to be added to the formulation, playing a variety of common roles of which are lyo-protectants, thermal stabilizers, and bulking agents. Lyo-protectants help maintain functionality of the active ingredients through the freezing and drying process by protecting the important molecules of the formulation. Thermal stabilizers are added to ensure the formulation has the right Tg or Te needed to fall in the necessary range required for successful LYO. Bulking agents can help provide an elegant structure and form a matrix that can support the active ingredients. Through our expertise, we can help you develop a product that meets you and your user’s needs.
Developing a formulation for lyophilization must account for several important factors. First, stability of the active ingredients must be considered, especially when it comes to biomolecules. For example, it may be important to work within specific pH ranges to maintain functionality for certain components. Besides functional considerations, critical final product quality attributes such as appearance, color, robustness, solubility, and residual moisture must also be considered. Finally, scientists must consider the thermal properties of a formulation ensuring its glass transition temperature or eutectic temperature is in a range suitable for LYO. To meet these targets, excipients are carefully selected to be added to the formulation, playing a variety of common roles of which are lyo-protectants, thermal stabilizers, and bulking agents. Lyo-protectants help maintain functionality of the active ingredients through the freezing and drying process by protecting the important molecules of the formulation. Thermal stabilizers are added to ensure the formulation has the right Tg or Te needed to fall in the necessary range required for successful LYO. Bulking agents can help provide an elegant structure and form a matrix that can support the active ingredients. Through our expertise, we can help you develop a product that meets you and your user’s needs.
